Carbon Chemical Symbol “C” Atomic Number 06
Carbon is one of the most important existing chemical in the world. It is a foundation for many chemical compounds, mixtures and steel and metallic alloys. The position of carbon in the periodic table is at group 14 or simply as IV-A. Carbon is the 6th important element in the periodic table. It is located amid boron i.e. “B” and nitrogen i.e. “N”. Carbon is found to be a very stable element. Since it is stable, it is occurred both on its own and in different naturally available compounds. Scientists name the basic 3 states of carbon and calls these as the amorphous, diamond, and graphite.
Occurrence: Carbon is a chemical element with symbol C and atomic number 6. It mostly lacks metallic attributes and it is having valency of four electrons. It could be seen on the Group 14 of the periodic table. There are three isotopes of Carbon which are 12C, 13C 14C. 12, 13 and 14 are different atomic masses which makes them isotopes. 12C, 13C are stable, while 14C is unstable due to excess nuclear energy.

Role of Carbon as Content in Alloy: Steel is fundamentally made up of iron and carbon alloyed with some other important elements for example Sulphur. The manipulative process of alloying is meant to alter the chemical elementary specification of alloy steel and enhance its properties over other types of steels or to adjust the properties to meet a set of requirements for a specific application. SUS304 steel, SPCC steel and UNS S17400 steel are some of the different alloys of steel having different level of carbon content in it.
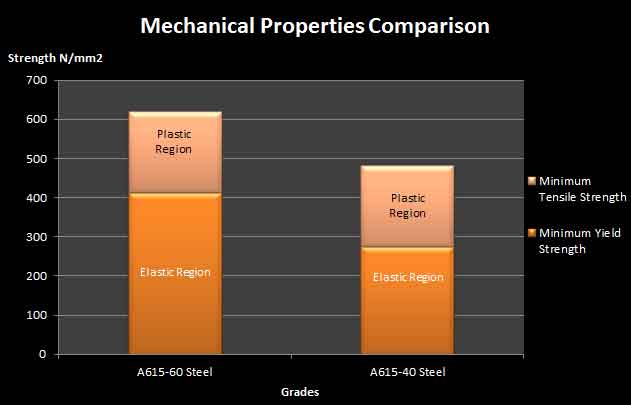
Carbon has a major impact as alloy on the mechanical properties of steel.
Carbon is the basic hardening content in steel.
Hardness as well as tensile strength rises with the increase of carbon in alloys. On the other hand the increase of carbon reduces the ductility and weldability of steel.
Physical Properties: Carbon is a soft, dull gray or black non-metal that you can easily scratch. The density of carbon as graphite is 2.267 g/mL, which means it will sink in water easily. For Graphite its density is from 2.25 g/cm³ and for Diamond it is 3.51 g/cm³. The melting point of graphite is 3500ºC and the extended boiling point is 4830ºC. Carbon is a unique element. It occurs in many states. Some examples of the pure form of carbon are coal and soot. It is soft and dull grey or black in color. One of the most important compounds of carbon is the charcoal; due to absence of air it is formed.
Carbon exists as 15th most abundant element in the earth and in universe due to mass it exists as fourth most abundant element. Carbon’s exists due to its unique ranges of organic compounds. It also contains unusual ability to form large molecule i.e. polymers. Carbon is the second most existing element in human body. It is due to Hydrocarbon compounds in which Carbon is the most important part, organic chemistry evolved.
Chemical Properties: The chemical properties of carbon depend on the crystallized structure of carbon. Chemical properties of Carbon as graphite are to burn from gaseous carbon to carbon dioxide. Carbon forms a very large number of organic compounds because it can form covalent bonding with itself and with other elements, hydrogen being most important.
Due to the amount of the carbon; living things contain, all organic things are carbon based. Each carbon atom can only form four single molecular bonds. The covalent bonds make it feasible for Carbon to bond long chain structures which are known as polymers or simple known as plastics.